プレスリリース
2022年11月3日 根寄生雑草被害防除に光明!
2022年11月2日 不安定な化学構造の植物ホルモンを植物内に大量蓄積させることに成功
2022年8月9日 植物の地下部でのコミュニケーションを可能にしている分子?
2022年7月13日 植物の繁栄を支える菌根菌共生の起源
2021年9月21日 根寄⽣雑草の寄⽣を制御する酵素の解明
2019年9月13日 病害寄生雑草ストライガの全ゲノム解読に成功
2017年8月18日 種子の寿命をコントロールする
2016年5月17日 植物の枝分かれ抑制ホルモンをつくる酵素を発見
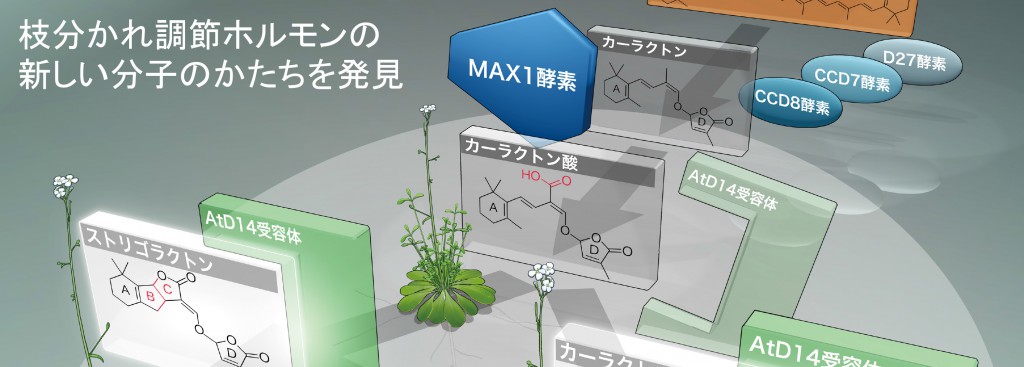
2014年11月25日 枝分かれ調節ホルモンの新しい分子のかたちを発見
2013年1月15日 植物生長ホルモン「ジベレリンA1」の唯一未解明の生合成酵素遺伝子を発見
論文等
2025年
73) Kawada K., Takahashi I., Saito T., Inayama T., Seto Y., Nomura T., Sasaki Y., Asami T., Yajima S., Ito S.* Optimization of the KK5 Scaffold and Biological Evaluation of KK5 Derivatives to Identify Potent Strigolactone Biosynthesis Inhibitors. J Agric Food Chem., in press, (2025) https://doi.org/10.1021/acs.jafc.5c01991
72) Hasegawa R., Ikeda M., Fujita K., Tanaka Y., Takasaki H., Kinugasa Y., Tachibana R., Yamagami A., Nomura T., Yano T., Mitsuda N., Nakano T., Ohme-Takagi M.* Arabidopsis homeodomain transcription factor BRASSINOSTEROID-RELATED HOMEOBOX 1 represses BR responses under stress conditions. J Exp Bot. eraf233, (2025) https://doi.org/10.1093/jxb/eraf233
2024年
71) Nomura T., Seto Y., Kyozuka J.* Unveiling the complexity of strigolactones: Exploring structural diversity, biosynthesis pathways and signaling mechanisms. J. Exp. Bot., 75: 1131-1133 (2024) https://doi.org/10.1093/jxb/erad412
野村崇人 テルペノイド系植物ホルモン生合成経路におけるシトクロムP450の機能解析に関する研究, 植物の生長調節, 59(1): 1-8 (2024)
2023年
70) Kameoka H.*, Shimazaki S., Mashiguchi K., Watanabe B., Komatsu A., Yoda A., Mizuno Y., Kodama K., Okamoto M., Nomura T., Yamaguchi S., Kyozuka J.* DIENELACTONE HYDROLASE LIKE PROTEIN1 negatively regulates the KAI2-ligand pathway in Marchantia polymorpha. Curr Biol. 33: 3505-3513.e5 (2023) https://doi.org/10.1016/j.cub.2023.06.083
69) Yoda A., Xie X., Yoneyama K., Miura K., McErlean C.S.P., Nomura T.* A stereoselective strigolactone biosynthesis catalyzed by a 2-oxoglutarate-dependent dioxygenase in sorghum. Plant Cell Physiol. 64: 1034-1045 (2023) https://doi.org/10.1093/pcp/pcad060
68) Wu S., Zhou A., Hiugano K., Yoda A., Xie X., Yamane K., Miura K., Nomura T.*, Li Y*. Identification of a Prunus MAX1 homolog as a unique strigol synthase. New Phytol. 239: 1819-1833 (2023) https://doi.org/10.1111/nph.19052
67) Kawada K., Saito T., Onoda S., Inayama T., Takahashi I., Seto Y., Nomura T., Sasaki Y., Asami T., Yajima S., Ito S.* Synthesis of carlactone derivatives to develop a novel inhibitor of strigolactone biosynthesis. ACS Omega 8(5): 13855–13862(2023) http://doi.org/10.1021/acsomega.3c00098
66) Ono A., Zhang J., Tanaka Y., Sato C., Yoda A., Ogata M., Nomura T., Suzuki T.* Heterologous expression of the lectin CmRlec from Cordyceps militaris (Cordycipitaceae, Ascomycota) in Escherichia coli. Biosci Biotechnol Biochem. 87(7): 742-746 (2023) doi: 10.1093/bbb/zbad045.
野村崇人 ストリゴラクトン研究の新しい展開, はじめに, 植物の生長調節, 58(2): 78-79 (2023)
野村崇人 植物特化代謝物の生合成研究, 植物細胞を利用した有用タンパク質の高生産, アグリバイオ, 7(9): 36-40 (2023)
2022年
65) Ito S., Braguy J., Wang JY., Yoda A., Fiorilli V., Takahashi I., Jamil M., Felemban A., Miyazaki S., Mazzarella T., Chen G.E., Shinozawa A., Balakrishna A., Berqdar L., Rajan C., Ali S., Haider I., Sasaki Y., Yajima S., Akiyama K., Lanfranco L., Zurbriggen M.D., Nomura T.*, Asami T.*, Al-Babili S.* Canonical strigolactones are not the major determinant of tillering but important rhizospheric signals in rice. Sci. Adv. 8(44): eadd1278 (2022) https://doi.org/10.1126/sciadv.add1278
64) Yata A., Nosaki S., Yoda A., Nomura T.*, Miura K.* Production and stably maintenance of strigolactone by transient expression of biosynthetic enzymes in Nicotiana benthamiana. Front. Plant Sci. 13:1027004 (2022) https://doi.org/10.3389/fpls.2022.1027004
62) Kodama K., Rich M.K., Yoda A, Shimazaki S., Xie X., Akiyama K., Mizuno Y., Komatsu A., Luo Y., Suzuki H., Kameoka H., Libourel C., Keller J., Sakakibara K., Nishiyama T., Nakagawa T., Mashiguchi K., Uchida K., Yoneyama K., Tanaka Y., Yamaguchi S., Shimamura M., Delaux P.-M.*, Nomura T.*, Kyozuka J.* An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 13: 3974 (2022) https://doi.org/10.1038/s41467-022-31708-3
61) Kusajima M., Fujita M., Soudthedlath K., Nakamura H., Yoneyama K., Nomura T., Akiyama K., Maruyama-Nakashita A., Asami T., Nakashita H.* Strigolactones modulate salicylic acid-mediated disease resistance in Arabidopsis thaliana. Int. J. Mol. Sci., 23: 5246 (2022) https://doi.org/10.3390/ijms23095246
60) Kyozuka J.*, Nomura T., Shimamura M. Origins and evolution of the dual functions of strigolactones as rhizosphere signaling molecules and plant hormones. Curr. Opin. Plant. Biol., 65: 102154 (2022) https://doi.org/10.1016/j.pbi.2021.102154
2021年
59) Yoda A., Mori N., Akiyama K., Kikuchi M., Xie X., Miura K., Yoneyama K., Sato-Izawa K., Yamaguchi S., Yoneyama Y., Nelson D.C., Nomura T.* Strigolactone biosynthesis catalyzed by cytochrome P450 and sulfotransferase in sorghum. New Phytol., 232: 1999-2010 (2021) https://doi.org/10.1111/nph.17737
経塚淳子*, 野村崇人 ストリゴラクトンの二面的機能, その起源と進化, 植物の生長調節, 56(2): 63-70 (2021) https://doi.org/10.18978/jscrp.56.2_63
2020年
58) Kawada K., Uchida Y., Takahashi I., Nomura T., Sasaki Y., Asami T., Yajima S., Ito S.* Triflumizole as a novel lead compound for strigolactone biosynthesis inhibitor. Molecules, 25(23): E5525 (2020) https://doi: 10.3390/molecules25235525
57) Yoneyama K.*, Akiyama K., Brewer P.B., Mori N., Kawano-Kawada M., Haruta S., Nishiwaki H., Yamauchi S., Xie X., Umehara M., Christine A. Beveridge C.A., Yoneyama K., Nomura T. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct, e00219 (2020) https://doi.org/10.1002/pld3.219
56) Yoneyama K.*, Xie X., Nomura T, Yoneyama K. Do phosphate and cytokinin interact to regulate strigolactone biosynthesis or act independently? Front. Plant Sci., 11: 438 (2020) https://doi.org/10.3389/fpls.2020.00438
55) Mori N., Sado A., Xie X., Yoneyama K., Asami K., Seto Y., Nomura T., Yamaguchi S., Yoneyama K., Akiyama K.* Chemical identification of 18-hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and noncanonical strigolactones in Lotus japonicus. Phytochem., 174: 112349 (2020) https://doi.org/10.1016/j.phytochem.2020.112349
54) Mori N., Nomura T., Akiyama K.* Identification of two oxygenase genes involved in the respective biosynthetic pathways of canonical and non‑canonical strigolactones in Lotus japonicus. Planta, 251: 40 (2020) https://doi.org/10.1007/s00425-019-03332-x
2019年
53) Yoshida S., Kim S., Wafula E.K., Tanskanen J., Kim Y., Honaas L., Yang Z., Spallek T., Conn11 C.E., Ichihashi Y., Cheong K., Cui1 S., Der J.P., Gundlach H., Jiao Y., Hori C., Ishida J.K., Kasahara H., Kiba T., Kim M., Koo N., Laohavisit A., Lee Y., Lumba S., McCourt P., Mortimer J.C., Mutuku J.M., Nomura T., Sasaki-Sekimoto Y., Seto Y., Wang Y., Wakatake T., Sakakibara H., Demura T., Yamaguchi S., Yoneyama K., Manabe R., Nelson D.C., Schulman A.H., Timko M.P., dePamphilis C.W., Choi D., Shirasu K.* Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr. Biol., 29: 3041-3052 (2019) https://doi.org/10.1016/j.cub.2019.07.086
52) Yoneyama K.*, Xie X., Yoneyama K., Nomura T., Takahashi I., Asami T., Mori N., Akiyama K., Kusajima M., Nakashita H. Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag. Sci., 75: 2353-2359 (2019) https://doi.org/10.1002/ps.5401
51) Xie X., Mori N., Yoneyama K., Nomura T., Uchida K., Yoneyama K., Akiyama K.* Lotuslactone, a non-canonical strigolactone from Lotus japonicus. Phytochem., 157: 200-205 (2019) https://doi.org/10.1016/j.phytochem.2018.10.034
2018年
50) Fàbregas N., Lozano-Elena F., Blasco-Escámez D., Tohge T., Martínez-Andújar C., Albacete A., Osorio S., Bustamante M., Riechmann J.L., Nomura T., Yokota T., Conesa A., Alfocea F.P., Fernie A.R., Caño-Delgado A.I.* Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun., 9: 4680 (2018) https://doi.org/10.1038/s41467-018-06861-3
49) Yoneyama K.*, Xie X., Yoneyama K., Kisugi T., Nomura T., Nakatani Y., Akiyama K., McErlean C.S.P. Which are major players, canonical or non-canonical strigolactones? J. Exp. Bot., 69: 2231-2239 (2018) https://doi.org/10.1093/jxb/ery090
48) Yoneyama K., Mori N., Stao T., Yoda A., Xie X., Okamoto M., Iwanaga M., Ohnishi T., Nishiwaki H., Asami T., Yokota T., Akiyama K., Yoneyama K., Nomura T.* Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytol., 218: 1522-1533 (2018) https://doi.org/10.1111/nph.15055
2017年
47) Sano N., Kim J.-S., Onda Y., Nomura T., Mochida K., Okamoto M., Seo M.* RNA-Seq using bulked recombinant inbred line populations uncovers the importance of brassinosteroid for seed longevity after priming treatments, Sci. Rep., 7: 8095 (2017) https://doi.org/10.1038/s41598-017-08116-5
46) Xie X., Kisugi T., Yoneyama K., Nomura T., Akiyama K., Uchida K., Yokota T., McErlean C.S.P., Yoneyama K.* Methyl zealactonoate, a novel germination stimulant for root parasitic weeds produced by maize. J. Pestic. Sci., 42: 58-61 (2017) https://doi.org/10.1584/jpestics.D16-103
45) Yokota T.*, Ohnishi T., Shibata K., Asahina M., Nomura T., Fujita T., Ishizaki K., Kohchi T. Occurrence of brassinosteroids in non-flowering land plants, liverwort, moss, lycophyte and fern. Phytochem., 136: 46-55 (2017) https://doi.org/10.1016/j.phytochem.2016.12.020
2016年
44) Brewer P.B., Yoneyama K., Filardo F., Meyers E., Scaffidi A., Frickey T., Akiyama K., Seto Y., Dun E.A., Cremer J.E., Kerr S.C., Waters M.T., Flematti G.R., Mason M.G., Weiller G., Yamaguchi S., Nomura T., Smith S.M., Yoneyama K., Beveridge C.A.* LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA, 113: 6301-6306 (2016) https://doi.org/10.1073/pnas.1601729113
43) Xie X., Yoneyama K., Kisugi T., Nomura T., Akiyama K., Asami T., Yoneyama K.* Structure- and stereospecific transport of strigolactones from roots to shoots. J. Pestic. Sci., 41: 55–58 (2016) https://doi.org/10.1584/jpestics.D16-009
2015年
42) Xie X., Yoneyama K., Kisugi T., Nomura T., Akiyama K., Asami T., Yoneyama K.* Strigolactones are transported from roots to shoots, although not through the xylem. J. Pestic. Sci., 40: 214–216 (2015) https://doi.org/10.1584/jpestics.D15-045
41) Yoneyama K., Arakawa R., Ishimoto K., Kim H.I., Kisugi T., Xie X., Nomura T., Kanampiu F., Yokota T., Ezawa T., Yoneyama K.* Difference in Striga-susceptibility is reflected in strigolactone secretion profile, but not in compatibility and host preference in arbuscular mycorrhizal symbiosis in two maize cultivars. New Phytol., 206: 983-989 (2015) https://doi.org/10.1111/nph.13375
40) Yoneyama K., Kisugi T., Xie X., Arakawa R., Ezawa T., Nomura T., Yoneyama K.* Shoot-derived signals other than auxin are involved in systemic regulation of strigolactone production in roots. Planta, 241: 687-698 (2015) https://doi.org/10.1007/s00425-014-2208-x
2014年
39) Abe S., Sado A., Tanaka K., Kisugi T., Asami K., Ota S., Kim H.I., Yoneyama K., Xie X., Ohnishi T., Seto Y., Yamaguchi S.*, Akiyama K.*, Yoneyama K., Nomura T.* Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA, 111: 18084-18089 (2014) https://doi.org/10.1073/pnas.1410801111
38) Khetkam P., Xie X., Kisugi T., Kim H.I., Yoneyama K., Kisugi T., Uchida K., Yokota T., Nomura T., Yoneyama K.* 7α- and 7β-Hydroxyorobanchyl acetate as germination stimulants for root parasitic weeds produced by cucumber. J. Pestic. Sci., 39: 121-126 (2014) https://doi.org/10.1584/jpestics.D14-038
37) Asahina M., Tamaki Y, Sakamoto T., Shibata K., Nomura T., Yokota T.* Blue light-promoted rice leaf bending and unrolling are due to up-regulated brassinosteroid biosynthesis genes accompanied by accumulation of castasterone. Phytochem., 104: 21-29 (2014) https://doi.org/10.1016/j.phytochem.2014.04.017
36) Kim H.I., Kisugi T., Khetkam P., Xie X., Yoneyama K., Uchida K., Yokota T., Nomura T., McErlean C.S.P., Yoneyama K.* Avenaol, a germination stimulant for root parasitic plants from Avena strigosa. Phytochemi., 103: 85-88 (2014) https://doi.org/10.1016/j.phytochem.2014.03.030
2013年
35) Nomura T., Magome H.*, Hanada A., Takeda-Kamiya N., Mander L.N., Kamiya Y., Yamaguchi S.* Functional analysis of Arabidopsis CYP714A1 and CYP714A2 reveals that they are distinct gibberellin modification enzymes. Plant Cell Physiol., 54: 1837-1851 (2013) https://doi.org/10.1093/pcp/pct125
34) Yoneyama K., Xie X., Kisugi T., Nomura T., Yoneyama K.* Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta, 238: 885-894 (2013) https://doi.org/10.1007/s00425-013-1943-8
33) Magome H., Nomura T., Hanada A., Takeda-Kamiya N., Ohnishi T., Shinma Y., Katsumata T., Kawaide H., Kamiya Y., Yamaguchi S.* CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice. Proc. Natl. Acad. Sci. USA, 110: 1947-1952 (2013) https://doi.org/10.1073/pnas.1215788110
32) Kisugi T., Xie X., Kim H.I., Yoneyama K., Sado A., Akiyama K., Hayashi H., Uchida K., Yokota T., Nomura T., Yoneyama K.* Strigone, isolation and identification as a natural strigolactone from Houttuynia cordata. Phytochem., 87: 60-64 (2013) https://doi.org/10.1016/j.phytochem.2012.11.013
31) Xie X., Yoneyama K., Kisugi T., Uchida K., Ito S., Akiyama K., Hayashi H., Yokota T., Nomura T., Yoneyama K.* Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant, 6: 153-163 (2013) https://doi.org/10.1093/mp/sss139
2012年以前
30) Yoneyama K., Xie X., Kim H., Kisugi T., Nomura T., Sekimoto H., Yokota T., Yoneyama K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation. Planta, 235: 1197-1207 (2012) https://doi.org/10.1007/s00425-011-1568-8
29) Yoneyama K., Xie X., Kisugi T., Nomura T., Sekimoto H., Yokota T., Yoneyama K. Characterization of strigolactones exuded by Asteraceae plants. Plant. Growth Regul., 65: 495-504 (2011) https://doi.org/10.1007/s10725-011-9620-z
28) Kim H.I., Xie X., Kim H.S., Chun J.C., Yoneyama K., Nomura T., Takeuchi Y., Yoneyama K. Structure-activity relationship of naturally occurring strigolactones in Orobanche minorseed germination stimulation. J. Pestic. Sci., 35: 344-347 (2010) https://doi.org/10.1584/jpestics.G10-17
27) Tanaka A., Nakagawa H., Tomita C., Shimatani Z., Ohtake M., Nomura T., Jiang C.-J., Dubouzet J. G., Kikuchi S., Sekimoto H., Yokota T., Asami T., Kamakura T., Mori M. BRASSINOSTEROID UPREGULATED 1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol., 151: 669-680 (2009)
26) Symons G.M., Smith J.J., Nomura T., Davies N.W., Yokota T. and Reid J.B. The hormonal regulation of de-etiolation. Planta, 227: 1115-1125 (2008)
25) Nomura T., Ueno M., Yamada Y., Takatsuto S., Takeuchi Y. and Yokota T. Roles of brassinosteroids and related mRNAs in pea seed growth and germination. Plant Physiol., 143: 1680-1688 (2007)
24) Jager C.E., Symons G.M., Nomura T., Yamada Y., Smith J.J., Yamaguchi S., Kamiya Y., Weller J.L., Yokota T. and Reid J.B. Characterisation of two brassinosteriod C-6 oxidase genes in pea. Plant Physiol., 143: 1894-1904 (2007)
23) Iino M., Nomura T., Tamaki Y., Yamada Y., Yoneyama K., Takeuchi Y., Mori M., Asami T., Nakano T. and Yokota T. Progesterone: its occurrence in plants and involvement in plant growth. Phytochem., 68: 1664-1673 (2007)
22) Zhu Y., Nomura T. (co-first author), Xu Y., Zhang Y., Peng Y., Mao B., Hanada A., Zhou H., Wang R., Li P., Zhu X., Mander L.N., Kamiya Y., Yamaguchi S. and He Z. ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell, 18: 442-456 (2006)
21) Nomura T. and Bishop G.J. Cytochrome P450s in plant steroid hormone synthesis and metabolism. Phytochem. Rev., 5: 421-432 (2006)
20) Bishop G.J., Nomura T., Yokota T., Montoya T., Castle J., Harrison K., Kushiro T., Kamiya Y., Yamaguchi., Bancos S., Szatmari A.M., Szekeres M. Dwarfism and cytochrome P450-mediated C-6 oxidation of plant steroid hormones. Biochem. Soc. Trans., 34: 1199-1201 (2006)
19) Ohnishi T., Nomura T., Watanabe B., Ohta D., Yokota T., Miyagawa H., Sakata K. and Mizutani M. Tomato cytochrome P450 CYP734A7 functions in brassinosteroid catabolism. Phytochem., 67: 1895-1906 (2006)
野村崇人 ブラシノステロイドの生合成および受容体遺伝子の単離と機能に関する研究, 植物の生長調節, 41: 11-16 (2006)
18) Nomura T., Kushiro T., Yokota T., Kamiya Y., Bishop G.J. and Yamaguchi S. The last reaction producing brassinolide is catalyzed by cytochrome P450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J. Biol. Chem., 280: 17873-17879 (2005)
17) Montoya T., Nomura T., Yokota T., Farrar K., Harrison K., Jones J.G.D., Kaneta T., Kamiya Y., Szekeres M. and Bishop G.J. Patterns of Dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. Plant J., 42: 262-269 (2005)
16) Nomura T., Jager C.E., Kitasaka Y., Takeuchi K., Fukami M., Yoneyama K., Matsushita Y., Nyunoya H., Takatsuto S., Fujioka S., Smith J.J., Kerckhoffs L.H.J., Reid J.B. and Yokota T. Brassinosteroid deficiency due to truncated steroid 5α-reductase causes dwarfism in the lk mutant of pea. Plant Physiol., 135: 2220-2229 (2004)
15) Nomura T., Bishop G.J., Kaneta T., Reid J.B., Chory J. and Yokota T. The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J., 36: 291-300 (2003)
14) Montoya T., Nomura T. (co-first author), Farrar K., Kaneta T., Yokota T. and Bishop G.J. Cloning the tomato Curl3 gene highlights the putative dual role of the leucine rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell, 14: 3163-3176 (2002)
13) Mori M., Nomura T., Ooka H., Ishizaka M., Yokota T., Sugimoto K., Okabe K., Kajiwara H., Satoh K., Yamamoto K., Hirochika H. and Kikuchi S. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol., 130: 1152-1161 (2002)
12) Bancos S., Nomura T., Sato T., Molnar G., Bishop G.J., Koncz C., Yokota T., Nagy F. and Szekeres M. Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol., 130: 504-513 (2002)
11) Shimada Y., Fujioka S., Miyauchi N., Kushiro M., Takatsuto S., Nomura T., Yokota T., Kamiya Y., Bishop G.J. and Yoshida S. Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol., 126: 770-779 (2001)
10) Nomura T., Sato T., Bishop G.J., Kamiya Y., Takatsuto S. and Yokota T. Accumulation of 6-deoxocathasterone and 6-deoxocastasterone in Arabidopsis, pea and tomato is suggestive of common rate-limiting steps in brassinosteroid biosynthesis. Phytochem., 57: 171-179 (2001)
9) Yokota T., Sato T., Takeuchi Y., Nomura T., Uno K., Watanabe T. and Takatsuto S. Roots and shoots of tomato produce 6-deoxo-28-norcathasterone, 6-deoxo-28-nortyphasterol and 6-deoxo-28-norcastasterone, possible precursors of 28-norcastasterone. Phytochem., 58: 233-238 (2001)
8) Watanabe T., Yokota T., Shibata K., Nomura T., Seto H. and Takatsuto S. Cryptolide, a new brassinolide catabolite with a 23-oxo group from Japanese cedar pollen/anther and its synthesis. J. Chem. Research(s), 18-19 (2000)
7) Bishop G.J., Nomura T., Yokota T., Harrison K., Noguchi T., Fujioka S., Takatsuto S., Jones J.D.G. and Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA, 96: 1761-1766 (1999)
6) Nomura T., Kitasaka Y., Takatsuto S., Reid J.B., Fukami M. and Yokota T. Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol., 119: 1517-1526 (1999)
5) Klahre U., Noguchi T., Fujioka S., Takatsuto S., Yokota T., Nomura T., Yoshida S. and Chua N.-H. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell, 10: 1677-1690 (1998)
4) Takatsuto S., Goto C., Noguchi T., Nomura T., Fujioka S. and Yokota T. Synthesis of deuterio-labelled 24-methylenecholesterol and related steroids. J. Chem. Research(s), 206-207 (1998)
3) Nomura T., Nakayama M., Reid J.B., Takeuchi Y. and Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol., 113: 31-37 (1997)
2) Yokota T., Nomura T. and Nakayama M. Identification of brassinosteroids that appear to be derived from campesterol and cholesterol in tomato shoots. Plant Cell Physiol., 38: 1291-1294 (1997)
1) Sekimoto H., Hoshi M., Nomura T. and Yokota T. Zinc deficiency affects the levels of endogenous gibberellins in Zea mays L. Plant Cell Physiol., 38: 1087-1090 (1997)
図書
Xie X., Yoneyama K., Nomura T, Yoneyama K.* Evaluation and quantification of natural strigolactones from root exudates. In Strigolactones (Springer). Methods Mol. Biol. 2309: 3-12 (2021)
野村崇人, 横田孝雄 第7章ブラシノステロイド, 新しい植物ホルモンの科学 第3版, 講談社 (2016)
野村崇人, 横田孝雄 第7章ブラシノステロイド, 新しい植物ホルモンの科学 第2版, 講談社 (2010)
特許
野村崇人、依田彬義、鈴木智大、タンパク質の発現方法およびタンパク質発現ベクター、特開2020-130167
Yamaguchi S., Nomura T., Magome H., Kamiya Y., Method for producing steviol synthetase gene and steviol, US20080271205 A1
山口信次郎, 野村崇人, 真籠洋, 神谷勇治 植物の形態調整方法(半わい性植物作出法), 特開2009-65886
山口信次郎, 野村崇人, 真籠洋, 神谷勇治 ステビオール合成酵素遺伝子及びステビオールの製造方法, 特開2008-237110
野村崇人, 久城哲夫, 山口信次郎, 神谷勇治, 横田孝雄, Gerard Bishop ブラシノライド合成酵素, 特開2006-109728
山口信次郎, 野村崇人, 神谷勇治 ジベレリン代謝酵素, 特開2006-340611